News Detail
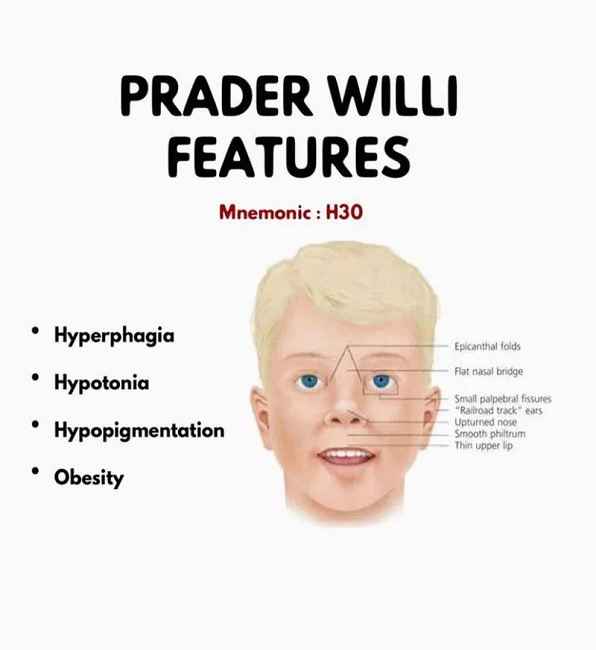
REDWOOD CITY, Calif., 26 MARCH 2025 (GLOBE NEWSWIRE): Soleno Therapeutics, Inc., a biopharmaceutical company developing novel therapeutics for the treatment of rare diseases, today announced that the U.S. Food and Drug Administration (FDA) has approved VYKAT XR (diazoxid......
View Details
Source : Soleno Therapeutics
Related News
- WB writes to Bihar and UP governments about spurious drugs makers (31-03-2025)
- Dabur to Drop ‘Anti-Inflammatory’, ‘Anti-Bacterial’, ‘Analgesic’ Claims from Toothpaste Labels by June 2025 (31-03-2025)
- Kangra: One person arrested from Delhi in drug case (30-03-2025)
- RMSCL to address drugs shortage using automated demand system (30-03-2025)
- Raids on chemist shops across Gujarat, 50 lakh Codeine Cough Syrup bottles seized (30-03-2025)
- Pluvicto can now be used before chemotherapy to treat a kind of metastatic prostate cancer (30-03-2025)
- HC asks Centre, Delhi govt to disclose regulatory mechanism for blood banks in city (30-03-2025)
- Meghalaya’s Citrus offers hope against antibiotic resistance (30-03-2025)
- Counterfeit anti-rabies vaccine circulating in major Indian cities (30-03-2025)
- Delhi HC awards ₹30.91 lakh costs & damages to Himalaya (29-03-2025)

