News Detail
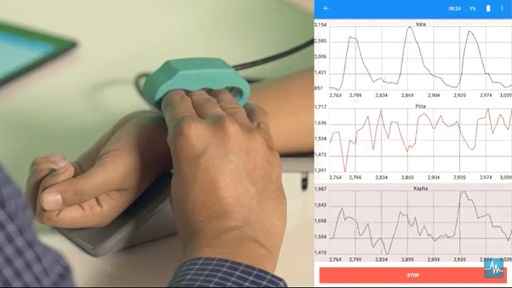
Pune, 6 Jan 2024: Nadi Tarangini (video), an indigenously developed pulse diagnostic tool, has become India’s first ayurvedic medical device to receive approval from the Central Drugs Standard Control Organization (CDSCO), the nati......
View Details
Source : The Hindu Businessline
Nadi Tarangini
ayurvedic
diagnostic device
CDSCO
Related News
- Punjab Police raids Baddi pharma unit (08-01-2025)
- Three drug smugglers arrested with 5200 narcotic capsules in Abohar (07-01-2025)
- FDA warning letter reveals poor quality control, inadequate handling of manufacturing errors at Viatris plant in Pithampur (07-01-2025)
- CSIR developed indigenous technology for paracetamol manufacturing (07-01-2025)
- Medical device sales hit Rs 8039.63 crore under PLI scheme (07-01-2025)
- Six new biomarkers could transform kidney injury monitoring and treatment (07-01-2025)
- IPC raises alarm on adverse reaction caused by Beta- blocker drugs (07-01-2025)
- Police launch probe into two infants’ deaths, kin blame vaccine (07-01-2025)
- 'HMPV does not spread like Covid-19': Expert debunks myths, explains precautions needed to combat virus (07-01-2025)
- Extension of revised Schedule M deadline fails to bring total relief to MSMEs (07-01-2025)

