News Detail
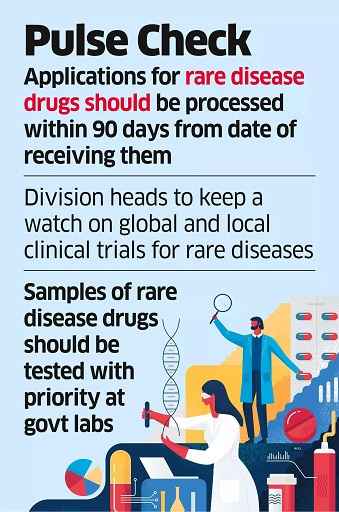
New Delhi, 13 Dec 2024: Fast-track clearances, waivers from clinical trials and faster access to drugs — the Union government has decided to prioritise arranging medicines and medical devices for patients in need via both global and local sourcin......
View Details
Source : News18
rare diseases
CDSCO
DCGI
WHO
health ministry
Related News
- Patanjali misleading medicine ads case: Next date of hearing on January 15 (14-12-2024)
- SC seeks replies from Centre, others on plea over snakebite treatment (14-12-2024)
- DoP re-opens application window for API CF sub-scheme (14-12-2024)
- Nursing colleges scam: MP HC orders removal of top officials (14-12-2024)
- 1.5L antibiotic tablets supplied to Nanded govt hosp spurious (14-12-2024)
- Didn't find sugar norms flouted in Nestle baby food: J P Nadda in LS (14-12-2024)
- Fake degrees seized from pvt institute in Hisar (13-12-2024)
- Health ministry assign testing labs for surgical and medical examination gloves (13-12-2024)
- Karnataka to restructure drugs control dept, strengthen procedures (13-12-2024)
- US FDA identifies cases of liver injury after treatment with Intercept's Ocaliva (obeticholic acid) (13-12-2024)

